Magnetic Moment Of The Loop
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagnets), permanent magnets, elementary particles (such every bit electrons), various molecules, and many astronomical objects (such every bit many planets, some moons, stars, etc).
More precisely, the term magnetic moment normally refers to a system'due south magnetic dipole moment, the component of the magnetic moment that can exist represented by an equivalent magnetic dipole: a magnetic northward and south pole separated by a very small distance. The magnetic dipole component is sufficient for small enough magnets or for large enough distances. College-society terms (such equally the magnetic quadrupole moment) may exist needed in addition to the dipole moment for extended objects.
The magnetic dipole moment of an object is readily defined in terms of the torque that the object experiences in a given magnetic field. The aforementioned applied magnetic field creates larger torques on objects with larger magnetic moments. The strength (and direction) of this torque depends non just on the magnitude of the magnetic moment but also on its orientation relative to the management of the magnetic field. The magnetic moment may be considered, therefore, to exist a vector. The direction of the magnetic moment points from the s to north pole of the magnet (inside the magnet).
The magnetic field of a magnetic dipole is proportional to its magnetic dipole moment. The dipole component of an object's magnetic field is symmetric nigh the direction of its magnetic dipole moment, and decreases every bit the inverse cube of the distance from the object.
Definition, units, and measurement [edit]
Definition [edit]
The magnetic moment can be divers as a vector relating the aligning torque on the object from an externally applied magnetic field to the field vector itself. The human relationship is given by:[ane]
where τ is the torque interim on the dipole, B is the external magnetic field, and m is the magnetic moment.
This definition is based on how one could, in principle, measure the magnetic moment of an unknown sample. For a current loop, this definition leads to the magnitude of the magnetic dipole moment equaling the product of the current times the expanse of the loop. Farther, this definition allows the calculation of the expected magnetic moment for whatsoever known macroscopic current distribution.
An alternative definition is useful for thermodynamics calculations of the magnetic moment. In this definition, the magnetic dipole moment of a system is the negative gradient of its intrinsic energy, U int , with respect to external magnetic field:
Generically, the intrinsic energy includes the cocky-field energy of the arrangement plus the energy of the internal workings of the system. For case, for a hydrogen atom in a 2p country in an external field, the self-field energy is negligible, so the internal energy is essentially the eigenenergy of the 2p state, which includes Coulomb potential energy and the kinetic energy of the electron. The interaction-field energy between the internal dipoles and external fields is not part of this internal energy.[ii]
Units [edit]
The unit for magnetic moment in International System of Units (SI) base units is A⋅one thousand2, where A is ampere (SI base unit of current) and m is meter (SI base unit of altitude). This unit has equivalents in other SI derived units including:[3] [4]
where N is newton (SI derived unit of force), T is tesla (SI derived unit of magnetic flux density), and J is joule (SI derived unit of energy).[5] Although torque (Due north·m) and free energy (J) are dimensionally equivalent, torques are never expressed in units of energy.[six]
In the CGS system, at that place are several different sets of electromagnetism units, of which the main ones are ESU, Gaussian, and EMU. Among these, there are two alternative (non-equivalent) units of magnetic dipole moment:
- (ESU)
- (Gaussian and EMU),
where statA is statamperes, cm is centimeters, erg is ergs, and G is gauss. The ratio of these 2 non-equivalent CGS units (EMU/ESU) is equal to the speed of light in gratuitous space, expressed in cm⋅s−ane.
All formulae in this article are right in SI units; they may need to be changed for use in other unit systems. For example, in SI units, a loop of current with current I and area A has magnetic moment IA (meet below), but in Gaussian units the magnetic moment is IA / c .
Other units for measuring the magnetic dipole moment include the Bohr magneton and the nuclear magneton.
Measurement [edit]
The magnetic moments of objects are typically measured with devices called magnetometers, though not all magnetometers mensurate magnetic moment: Some are configured to measure magnetic field instead. If the magnetic field surrounding an object is known well enough, though, and so the magnetic moment can be calculated from that magnetic field.
Relation to magnetization [edit]
The magnetic moment is a quantity that describes the magnetic strength of an entire object. Sometimes, though, information technology is useful or necessary to know how much of the internet magnetic moment of the object is produced by a particular portion of that magnet. Therefore, it is useful to define the magnetization field Yard as:
where m ΔV and V Δ5 are the magnetic dipole moment and volume of a sufficiently small portion of the magnet ΔV . This equation is frequently represented using derivative notation such that
where dm is the uncomplicated magnetic moment and dFive is the volume element. The net magnetic moment of the magnet m therefore is
where the triple integral denotes integration over the book of the magnet. For uniform magnetization (where both the magnitude and the direction of 1000 is the same for the entire magnet (such as a direct bar magnet) the last equation simplifies to:
where V is the volume of the bar magnet.
The magnetization is often non listed as a material parameter for commercially bachelor ferromagnetic materials, though. Instead the parameter that is listed is residual flux density (or remanence), denoted B r . The formula needed in this case to calculate m in (units of A⋅mtwo) is:
- ,
where:
- B r is the residuum flux density, expressed in teslas.
- V is the volume of the magnet (in yard3).
- μ 0 is the permeability of vacuum ( 4π×x−7 H/m).[7]
Models [edit]
The preferred classical explanation of a magnetic moment has changed over fourth dimension. Before the 1930s, textbooks explained the moment using hypothetical magnetic point charges. Since then, well-nigh have divers it in terms of Ampèrian currents.[8] In magnetic materials, the crusade of the magnetic moment are the spin and orbital angular momentum states of the electrons, and varies depending on whether atoms in one region are aligned with atoms in another.
Magnetic pole model [edit]
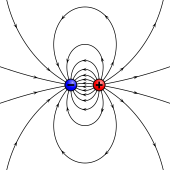
An electrostatic analog for a magnetic moment: two opposing charges separated past a finite distance.
The sources of magnetic moments in materials can exist represented by poles in illustration to electrostatics. This is sometimes known equally the Gilbert model.[ix] In this model, a small magnet is modeled by a pair of fictitious magnetic monopoles of equal magnitude merely opposite polarity. Each pole is the source of magnetic forcefulness which weakens with distance. Since magnetic poles always come in pairs, their forces partially cancel each other because while i pole pulls, the other repels. This counterfoil is greatest when the poles are close to each other i.e. when the bar magnet is short. The magnetic force produced by a bar magnet, at a given point in space, therefore depends on ii factors: the force p of its poles (magnetic pole strength), and the vector separating them. The magnetic dipole moment yard is related to the fictitious poles as[eight]
It points in the management from Due south to North pole. The illustration with electric dipoles should non be taken besides far because magnetic dipoles are associated with angular momentum (run across Relation to angular momentum). Withal, magnetic poles are very useful for magnetostatic calculations, particularly in applications to ferromagnets.[8] Practitioners using the magnetic pole approach by and large represent the magnetic field past the irrotational field H , in illustration to the electrical field Due east .
Amperian loop model [edit]

The Amperian loop model: A current loop (ring) that goes into the page at the x and comes out at the dot produces a B -field (lines). The north pole is to the right and the south to the left.
After Hans Christian Ørsted discovered that electric currents produce a magnetic field and André-Marie Ampère discovered that electrical currents attract and repel each other like to magnets, it was natural to hypothesize that all magnetic fields are due to electrical current loops. In this model developed past Ampère, the elementary magnetic dipole that makes upwardly all magnets is a sufficiently pocket-sized amperian loop of electric current I. The dipole moment of this loop is
where Southward is the area of the loop. The direction of the magnetic moment is in a direction normal to the expanse enclosed past the current consistent with the direction of the current using the right paw rule.
Localized electric current distributions [edit]
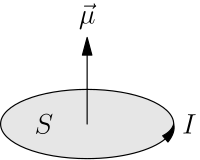
Moment of a planar current having magnitude I and enclosing an area S
The magnetic dipole moment can be calculated for a localized (does not extend to infinity) current distribution assuming that we know all of the currents involved. Conventionally, the derivation starts from a multipole expansion of the vector potential. This leads to the definition of the magnetic dipole moment every bit:
where × is the vector cross product, r is the position vector, and j is the electric current density and the integral is a volume integral.[10] When the current density in the integral is replaced by a loop of current I in a airplane enclosing an expanse Due south then the book integral becomes a line integral and the resulting dipole moment becomes
which is how the magnetic dipole moment for an Amperian loop is derived.
Practitioners using the current loop model generally represent the magnetic field by the solenoidal field B , analogous to the electrostatic field D .
Magnetic moment of a solenoid [edit]
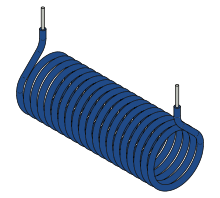
A generalization of the above current loop is a coil, or solenoid. Its moment is the vector sum of the moments of private turns. If the solenoid has N identical turns (unmarried-layer winding) and vector area S ,
Breakthrough mechanical model [edit]
When computing the magnetic moments of materials or molecules on the microscopic level it is oftentimes user-friendly to utilize a third model for the magnetic moment that exploits the linear relationship between the athwart momentum and the magnetic moment of a particle. While this relation is straight forward to develop for macroscopic currents using the amperian loop model (see below), neither the magnetic pole model nor the amperian loop model truly represents what is occurring at the atomic and molecular levels. At that level breakthrough mechanics must be used. Fortunately, the linear relationship betwixt the magnetic dipole moment of a particle and its angular momentum nevertheless holds, although it is dissimilar for each particle. Further, care must exist used to distinguish betwixt the intrinsic angular momentum (or spin) of the particle and the particle'southward orbital angular momentum. See below for more details.
Effects of an external magnetic field [edit]
Torque on a moment [edit]
The torque τ on an object having a magnetic dipole moment m in a compatible magnetic field B is:
- .
This is valid for the moment due to any localized current distribution provided that the magnetic field is compatible. For non-compatible B the equation is also valid for the torque most the center of the magnetic dipole provided that the magnetic dipole is modest enough.[xi]
An electron, nucleus, or atom placed in a compatible magnetic field will precess with a frequency known as the Larmor frequency. See Resonance.
Force on a moment [edit]
A magnetic moment in an externally produced magnetic field has a potential energy U:
In a example when the external magnetic field is non-uniform, there will be a strength, proportional to the magnetic field gradient, acting on the magnetic moment itself. There are two expressions for the force acting on a magnetic dipole, depending on whether the model used for the dipole is a current loop or ii monopoles (analogous to the electric dipole).[12] The force obtained in the case of a current loop model is
- .
Assuming existence of magnetic monopole, the strength is modified as follows:
In the example of a pair of monopoles beingness used (i.e. electrical dipole model), the forcefulness is
- .
And one can be put in terms of the other via the relation
- .
In all these expressions m is the dipole and B is the magnetic field at its position. Annotation that if in that location are no currents or time-varying electrical fields or magnetic charge, ∇×B = 0, ∇·B = 0 and the two expressions agree.
Relation to Energy [edit]
One can chronicle the magnetic moment of a system to the complimentary energy of that organisation.[13] In a uniform magnetic field B , the free energy F can be related to the magnetic moment M of the system as
where Southward is the entropy of the organisation and T is the temperature. Therefore, the magnetic moment tin can also be divers in terms of the free energy of a system as
.
Magnetism [edit]
In add-on, an applied magnetic field tin can change the magnetic moment of the object itself; for example by magnetizing it. This miracle is known every bit magnetism. An practical magnetic field can flip the magnetic dipoles that make up the textile causing both paramagnetism and ferromagnetism. Additionally, the magnetic field tin affect the currents that create the magnetic fields (such equally the atomic orbits) which causes diamagnetism.
Furnishings on environment [edit]
Magnetic field of a magnetic moment [edit]
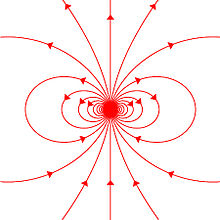
Magnetic field lines effectually a "magnetostatic dipole". The magnetic dipole itself is located in the center of the effigy, seen from the side, and pointing upward.
Any system possessing a net magnetic dipole moment m volition produce a dipolar magnetic field (described below) in the space surrounding the organization. While the net magnetic field produced past the system can also take higher-social club multipole components, those will drop off with distance more rapidly, so that merely the dipole component will dominate the magnetic field of the system at distances far away from it.
The magnetic field of a magnetic dipole depends on the strength and direction of a magnet's magnetic moment but drops off as the cube of the altitude such that:
where is the magnetic field produced by the magnet and is a vector from the heart of the magnetic dipole to the location where the magnetic field is measured. The inverse cube nature of this equation is more than readily seen past expressing the location vector as the production of its magnitude times the unit vector in its management ( ) so that:
The equivalent equations for the magnetic -field are the same except for a multiplicative cistron of μ 0 = fourπ ×10−7 H/thousand, where μ 0 is known as the vacuum permeability. For case:
Forces between two magnetic dipoles [edit]
As discussed before, the strength exerted by a dipole loop with moment m 1 on some other with moment g ii is
where B 1 is the magnetic field due to moment m 1 . The event of calculating the slope is[14] [15]
where r̂ is the unit of measurement vector pointing from magnet 1 to magnet 2 and r is the distance. An equivalent expression is[15]
The force interim on m 1 is in the opposite direction.
Torque of ane magnetic dipole on another [edit]
The torque of magnet 1 on magnet 2 is
Theory underlying magnetic dipoles [edit]
The magnetic field of any magnet tin can be modeled by a series of terms for which each term is more complicated (having finer angular detail) than the one before it. The commencement 3 terms of that serial are called the monopole (represented past an isolated magnetic n or south pole) the dipole (represented past two equal and contrary magnetic poles), and the quadrupole (represented past four poles that together course two equal and opposite dipoles). The magnitude of the magnetic field for each term decreases progressively faster with distance than the previous term, so that at big enough distances the first not-nix term will dominate.
For many magnets the first not-zero term is the magnetic dipole moment. (To date, no isolated magnetic monopoles have been experimentally detected.) A magnetic dipole is the limit of either a current loop or a pair of poles as the dimensions of the source are reduced to zero while keeping the moment constant. As long every bit these limits only utilize to fields far from the sources, they are equivalent. However, the two models give different predictions for the internal field (see below).
Magnetic potentials [edit]
Traditionally, the equations for the magnetic dipole moment (and college guild terms) are derived from theoretical quantities called magnetic potentials[16] which are simpler to deal with mathematically than the magnetic fields.
In the magnetic pole model, the relevant magnetic field is the demagnetizing field . Since the demagnetizing portion of does non include, by definition, the role of due to gratuitous currents, in that location exists a magnetic scalar potential such that
- .
In the amperian loop model, the relevant magnetic field is the magnetic induction . Since magnetic monopoles do not exist, in that location exists a magnetic vector potential such that
Both of these potentials tin can be calculated for any arbitrary current distribution (for the amperian loop model) or magnetic charge distribution (for the magnetic charge model) provided that these are limited to a small plenty region to requite:
where is the current density in the amperian loop model, is the magnetic pole strength density in illustration to the electric charge density that leads to the electric potential, and the integrals are the volume (triple) integrals over the coordinates that make up . The denominators of these equation can exist expanded using the multipole expansion to give a serial of terms that have larger of power of distances in the denominator. The offset nonzero term, therefore, volition dominate for large distances. The first non-naught term for the vector potential is:
where is:
where × is the vector cantankerous product, r is the position vector, and j is the electric electric current density and the integral is a volume integral.
In the magnetic pole perspective, the first non-zero term of the scalar potential is
Hither may be represented in terms of the magnetic pole strength density but is more usefully expressed in terms of the magnetization field as:
The same symbol is used for both equations since they produce equivalent results outside of the magnet.
External magnetic field produced by a magnetic dipole moment [edit]
The magnetic flux density for a magnetic dipole in the amperian loop model, therefore, is
Further, the magnetic field strength is
Internal magnetic field of a dipole [edit]
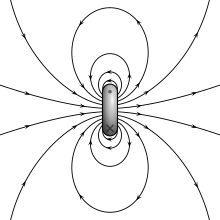
The magnetic field of a electric current loop
The two models for a dipole (current loop and magnetic poles) requite the same predictions for the magnetic field far from the source. However, within the source region, they give different predictions. The magnetic field betwixt poles (see effigy for Magnetic pole definition) is in the opposite direction to the magnetic moment (which points from the negative charge to the positive accuse), while inside a current loop information technology is in the same direction (encounter the figure to the correct). The limits of these fields must also be dissimilar equally the sources compress to goose egg size. This distinction only matters if the dipole limit is used to calculate fields within a magnetic fabric.[8]
If a magnetic dipole is formed by making a current loop smaller and smaller, but keeping the production of current and expanse constant, the limiting field is
Different the expressions in the previous section, this limit is correct for the internal field of the dipole.[8] [17]
If a magnetic dipole is formed by taking a "n pole" and a "south pole", bringing them closer and closer together but keeping the product of magnetic pole accuse and altitude constant, the limiting field is[8]
These fields are related past B = μ 0(H + M), where G(r) = m δ(r) is the magnetization.
Relation to angular momentum [edit]
The magnetic moment has a close connection with angular momentum called the gyromagnetic effect. This result is expressed on a macroscopic scale in the Einstein–de Haas effect, or "rotation by magnetization," and its inverse, the Barnett effect, or "magnetization by rotation."[1] Further, a torque practical to a relatively isolated magnetic dipole such as an atomic nucleus tin cause it to precess (rotate about the axis of the applied field). This phenomenon is used in nuclear magnetic resonance.
Viewing a magnetic dipole every bit current loop brings out the close connectedness between magnetic moment and angular momentum. Since the particles creating the electric current (past rotating around the loop) accept accuse and mass, both the magnetic moment and the angular momentum increase with the rate of rotation. The ratio of the ii is called the gyromagnetic ratio or so that:[18] [nineteen]
where is the angular momentum of the particle or particles that are creating the magnetic moment.
In the amperian loop model, which applies for macroscopic currents, the gyromagnetic ratio is i half of the accuse-to-mass ratio. This can be shown as follows. The angular momentum of a moving charged particle is defined as:
where μ is the mass of the particle and v is the particle's velocity. The angular momentum of the very large number of charged particles that make up a current therefore is:
where ρ is the mass density of the moving particles. By convention the management of the cross product is given by the right-hand dominion.[20]
This is like to the magnetic moment created by the very large number of charged particles that make upwards that current:
where and is the charge density of the moving charged particles.
Comparing the two equations results in:
where is the accuse of the particle and is the mass of the particle.
Even though atomic particles cannot exist accurately described as orbiting (and spinning) charge distributions of uniform charge-to-mass ratio, this general trend can exist observed in the atomic world so that:
where the g-factor depends on the particle and configuration. For example the g-factor for the magnetic moment due to an electron orbiting a nucleus is one while the one thousand-factor for the magnetic moment of electron due to its intrinsic angular momentum (spin) is a little larger than 2. The g-factor of atoms and molecules must account for the orbital and intrinsic moments of its electrons and possibly the intrinsic moment of its nuclei likewise.
In the atomic world the athwart momentum (spin) of a particle is an integer (or half-integer in the instance of spin) multiple of the reduced Planck abiding ħ. This is the basis for defining the magnetic moment units of Bohr magneton (assuming charge-to-mass ratio of the electron) and nuclear magneton (assuming accuse-to-mass ratio of the proton). See electron magnetic moment and Bohr magneton for more details.
Atoms, molecules, and uncomplicated particles [edit]
Fundamentally, contributions to whatsoever arrangement's magnetic moment may come from sources of two kinds: motility of electric charges, such every bit electric currents; and the intrinsic magnetism of elementary particles, such equally the electron.
Contributions due to the sources of the first kind can be calculated from knowing the distribution of all the electric currents (or, alternatively, of all the electric charges and their velocities) inside the system, by using the formulas beneath. On the other hand, the magnitude of each uncomplicated particle's intrinsic magnetic moment is a fixed number, often measured experimentally to a slap-up precision. For example, whatever electron'south magnetic moment is measured to be −9.284764 ×x−24 J/T.[21] The management of the magnetic moment of any elementary particle is entirely adamant by the direction of its spin, with the negative value indicating that any electron'south magnetic moment is antiparallel to its spin.
The net magnetic moment of whatever organization is a vector sum of contributions from one or both types of sources. For case, the magnetic moment of an atom of hydrogen-ane (the lightest hydrogen isotope, consisting of a proton and an electron) is a vector sum of the following contributions:
- the intrinsic moment of the electron,
- the orbital movement of the electron effectually the proton,
- the intrinsic moment of the proton.
Similarly, the magnetic moment of a bar magnet is the sum of the contributing magnetic moments, which include the intrinsic and orbital magnetic moments of the unpaired electrons of the magnet's material and the nuclear magnetic moments.
Magnetic moment of an cantlet [edit]
For an cantlet, private electron spins are added to get a full spin, and individual orbital angular momenta are added to go a total orbital angular momentum. These two so are added using athwart momentum coupling to get a total angular momentum. For an atom with no nuclear magnetic moment, the magnitude of the atomic dipole moment, , is and then[22]
where j is the total angular momentum breakthrough number, g J is the Landé g-factor, and μ B is the Bohr magneton. The component of this magnetic moment forth the direction of the magnetic field is then[23]
The negative sign occurs because electrons accept negative charge.
The integer grand (non to be confused with the moment, ) is called the magnetic breakthrough number or the equatorial quantum number, which tin take on any of iij + 1 values:[24]
Due to the angular momentum, the dynamics of a magnetic dipole in a magnetic field differs from that of an electric dipole in an electric field. The field does exert a torque on the magnetic dipole tending to align it with the field. However, torque is proportional to rate of change of angular momentum, so precession occurs: the direction of spin changes. This behavior is described by the Landau–Lifshitz–Gilbert equation:[25] [26]
where γ is the gyromagnetic ratio, m is the magnetic moment, λ is the damping coefficient and H eff is the effective magnetic field (the external field plus whatsoever self-induced field). The showtime term describes precession of the moment nigh the effective field, while the second is a damping term related to dissipation of free energy caused past interaction with the surroundings.
Magnetic moment of an electron [edit]
Electrons and many uncomplicated particles also have intrinsic magnetic moments, an explanation of which requires a breakthrough mechanical treatment and relates to the intrinsic athwart momentum of the particles as discussed in the article Electron magnetic moment. Information technology is these intrinsic magnetic moments that requite ascension to the macroscopic effects of magnetism, and other phenomena, such as electron paramagnetic resonance.
The magnetic moment of the electron is
where μ B is the Bohr magneton, S is electron spin, and the g-factor yard S is two according to Dirac's theory, simply due to breakthrough electrodynamic effects it is slightly larger in reality: ii.002319 304 36 . The deviation from two is known as the dissonant magnetic dipole moment.
Again it is important to detect that m is a negative abiding multiplied by the spin, and so the magnetic moment of the electron is antiparallel to the spin. This can be understood with the following classical picture: if we imagine that the spin angular momentum is created by the electron mass spinning around some centrality, the electric electric current that this rotation creates circulates in the reverse direction, considering of the negative accuse of the electron; such current loops produce a magnetic moment which is antiparallel to the spin. Hence, for a positron (the anti-particle of the electron) the magnetic moment is parallel to its spin.
Magnetic moment of a nucleus [edit]
The nuclear system is a complex physical organization consisting of nucleons, i.e., protons and neutrons. The quantum mechanical properties of the nucleons include the spin among others. Since the electromagnetic moments of the nucleus depend on the spin of the individual nucleons, 1 can look at these properties with measurements of nuclear moments, and more specifically the nuclear magnetic dipole moment.
Most common nuclei exist in their footing country, although nuclei of some isotopes have long-lived excited states. Each energy state of a nucleus of a given isotope is characterized by a well-defined magnetic dipole moment, the magnitude of which is a fixed number, often measured experimentally to a great precision. This number is very sensitive to the individual contributions from nucleons, and a measurement or prediction of its value tin reveal important information almost the content of the nuclear wave role. At that place are several theoretical models that predict the value of the magnetic dipole moment and a number of experimental techniques aiming to carry out measurements in nuclei along the nuclear chart.
Magnetic moment of a molecule [edit]
Any molecule has a well-defined magnitude of magnetic moment, which may depend on the molecule's energy state. Typically, the overall magnetic moment of a molecule is a combination of the following contributions, in the order of their typical strength:
- magnetic moments due to its unpaired electron spins (paramagnetic contribution), if whatsoever
- orbital movement of its electrons, which in the footing state is often proportional to the external magnetic field (diamagnetic contribution)
- the combined magnetic moment of its nuclear spins, which depends on the nuclear spin configuration.
Examples of molecular magnetism [edit]
- The dioxygen molecule, Otwo, exhibits strong paramagnetism, due to unpaired spins of its outermost two electrons.
- The carbon dioxide molecule, CO2, by and large exhibits diamagnetism, a much weaker magnetic moment of the electron orbitals that is proportional to the external magnetic field. The nuclear magnetism of a magnetic isotope such every bit 13C or 17O will contribute to the molecule'due south magnetic moment.
- The dihydrogen molecule, Htwo, in a weak (or zero) magnetic field exhibits nuclear magnetism, and tin be in a para- or an ortho- nuclear spin configuration.
- Many transition metal complexes are magnetic. The spin-but formula is a good get-go approximation for high-spin complexes of first-row transition metals.[27]
-
Number of
unpaired
electronsSpin-but
moment
( μ B )1 1.73 2 ii.83 3 3.87 four four.90 5 five.92
Simple particles [edit]
In atomic and nuclear physics, the Greek symbol μ represents the magnitude of the magnetic moment, often measured in Bohr magnetons or nuclear magnetons, associated with the intrinsic spin of the particle and/or with the orbital motion of the particle in a organisation. Values of the intrinsic magnetic moments of some particles are given in the tabular array beneath:
-
Intrinsic magnetic moments and spins
of some uncomplicated particles[28]Particle
name (symbol)Magnetic
dipole moment
(10−27 J⋅T−1)Spin
quantum number
(dimensionless)electron (eastward−) −9284.764 one / 2 proton (H+) –0 0 14.106067 1 / ii neutron (northward) 0 00 −9.66236 1 / ii muon (μ−) 0 0 −44.904478 1 / ii deuteron (iiH+) –0 00 4.3307346 one triton (3H+) –0 0 fifteen.046094 one / 2 helion (3He++) 0 0 −ten.746174 1 / 2 alpha particle (fourHe++) –0 000 0
For the relation betwixt the notions of magnetic moment and magnetization see magnetization.
See also [edit]
- Moment (physics)
- Electric dipole moment
- Toroidal dipole moment
- Magnetic susceptibility
- Orbital magnetization
- Magnetic dipole–dipole interaction
- Electron magnetic moment
- Nucleon magnetic moment
References and notes [edit]
- ^ a b Cullity, B. D.; Graham, C. D. (2008). Introduction to Magnetic Materials (2nd ed.). Wiley-IEEE Press. p. 103. ISBN978-0-471-47741-9.
- ^ Encounter, for example, Callen, Herbert B. (1985). Thermodynamics and an Introduction to Thermostatistics (2nd ed.). John Wiley & Sons. p. 200. ISBN978-0-471-86256-7. where the relevant U is U[Bdue east].
- ^ "Magnetic units". IEEE Magnetics. Retrieved 19 February 2016.
- ^ Mohr, Peter J.; Newell, David B.; Taylor, Barry N. (21 Jul 2015). "CODATA Recommended Values of the Fundamental Physical Constants: 2014". Reviews of Modern Physics. 88 (3): 035009. arXiv:1507.07956. Bibcode:2016RvMP...88c5009M. doi:10.1103/RevModPhys.88.035009. S2CID 1115862.
- ^ Le Système international d'unités [The International Arrangement of Units] (PDF) (in French and English) (9th ed.), International Bureau of Weights and Measures, 2019, ISBN978-92-822-2272-0 , pp. 20-21
- ^ Le Système international d'unités [The International System of Units] (PDF) (in French and English language) (9th ed.), International Bureau of Weights and Measures, 2019, ISBN978-92-822-2272-0 , p. 23
- ^ "1000&J Magnetics – Glossary". www.kjmagnetics.com.
- ^ a b c d e f Brown, William Fuller Jr. (1962). Magnetostatic Principles in Ferromagnetism. North-Holland.
- ^ Griffiths, David J. (1999). Introduction to Electrodynamics (3rd ed.). Prentice Hall. p. 258. ISBN978-0-13-805326-0. OCLC 40251748.
- ^ Jackson, John David (1975). "5.six Magnetic fields of a Localized Electric current Distribution, Magnetic Moment". Classical Electrodynamics. Vol. 2. ISBN978-0-471-43132-nine.
- ^ Griffiths, David J. (1999). Introduction to Electrodynamics (third ed.). Prentice Hall. p. 257. ISBN978-0138053260.
- ^ Boyer, Timothy H. (1988). "The Force on a Magnetic Dipole". Am. J. Phys. 56 (8): 688–692. Bibcode:1988AmJPh..56..688B. doi:10.1119/1.15501.
- ^ Landau, L. D.; Lifshitz, Eastward. M.; Pitaevskii, 50. P. (January 15, 1984). Electrodynamics of Continuous Media: Book 8 (Class of Theoretical Physics) (2 ed.). Butterworth-Heinemann. p. 130. ISBN978-0750626347.
- ^ Furlani, Edward P. (2001). Permanent Magnet and Electromechanical Devices: Materials, Analysis, and Applications. Bookish Printing. p. 140. ISBN978-0-12-269951-ane.
- ^ a b Yung, K. Westward.; Landecker, P. B.; Villani, D. D. (1998). "An Analytic Solution for the Strength between Two Magnetic Dipoles" (PDF). Magnetic and Electrical Separation. 9: 39–52. doi:10.1155/1998/79537 . Retrieved Nov 24, 2012.
- ^ Jackson, John David (1975). "5.half dozen". Classical electrodynamics (2nd ed.). New York: Wiley. ISBN9780471431329.
- ^ Jackson, John David (1975). Classical electrodynamics (2d ed.). New York: Wiley. p. 184. ISBN978-0-471-43132-9.
- ^ Krey, Uwe; Owen, Anthony (2007). Basic Theoretical Physics. Springer. pp. 151–152. ISBN978-3-540-36804-5.
- ^ Buxton, Richard B. (2002). Introduction to functional magnetic resonance imaging. Cambridge University Press. p. 136. ISBN978-0-521-58113-4.
- ^ Feynman, Richard P.; Leighton, Robert B.; Sands, Matthew (2006). The Feynman Lectures on Physics. Vol. ii. pp. 13–12. ISBN978-0-8053-9045-2.
- ^ "CODATA Value: electron magnetic moment". physics.nist.gov.
- ^ Tilley, R. J. D. (2004). Understanding Solids. John Wiley and Sons. p. 368. ISBN978-0-470-85275-0.
- ^ Tipler, Paul Allen; Llewellyn, Ralph A. (2002). Modern Physics (quaternary ed.). Macmillan. p. 310. ISBN978-0-7167-4345-three.
- ^ Crowther, J.A. (1949). Ions, Electrons, and Ionizing Radiations (8th ed.). London: Edward Arnold. p. 270.
- ^ Rice, Stuart Alan (2004). Advances in chemical physics. Wiley. pp. 208ff. ISBN978-0-471-44528-ix.
- ^ Steiner, Marcus (2004). Micromagnetism and Electrical Resistance of Ferromagnetic Electrodes for Spin Injection Devices. Cuvillier Verlag. p. 6. ISBN978-iii-86537-176-8.
- ^ Figgis, B.North.; Lewis, J. (1960). "The magnetochemistry of complex compounds". In Lewis, J.; Wilkins, R.G. (eds.). Mod Coordination Chemical science: Principles and methods. New York: Interscience. pp. 405–407.
- ^ "Search results matching 'magnetic moment'". CODATA internationally recommended values of the Cardinal Physical Constants. National Institute of Standards and Technology. Retrieved 11 May 2012.
External links [edit]
- Bowtell, Richard (2009). "μ – Magnetic Moment". Sixty Symbols. Brady Haran for the University of Nottingham.
Magnetic Moment Of The Loop,
Source: https://en.wikipedia.org/wiki/Magnetic_moment
Posted by: harristhentlyst.blogspot.com
















































![{\displaystyle \mathbf {B} (\mathbf {r} )={\frac {\mu _{0}}{4\pi }}\left[{\frac {3\mathbf {\hat {r}} (\mathbf {\hat {r}} \cdot \mathbf {m} )-\mathbf {m} }{|\mathbf {r} |^{3}}}+{\frac {8\pi }{3}}\mathbf {m} \delta (\mathbf {r} )\right].}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b47f91d20595386326b2945ac17533fd823321db)
![{\displaystyle \mathbf {H} (\mathbf {r} )={\frac {1}{4\pi }}\left[{\frac {3\mathbf {\hat {r}} (\mathbf {\hat {r}} \cdot \mathbf {m} )-\mathbf {m} }{|\mathbf {r} |^{3}}}-{\frac {4\pi }{3}}\mathbf {m} \delta (\mathbf {r} )\right].}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a1b635de5e52b7fd68571a72866d50392b0b7213)




















0 Response to "Magnetic Moment Of The Loop"
Post a Comment